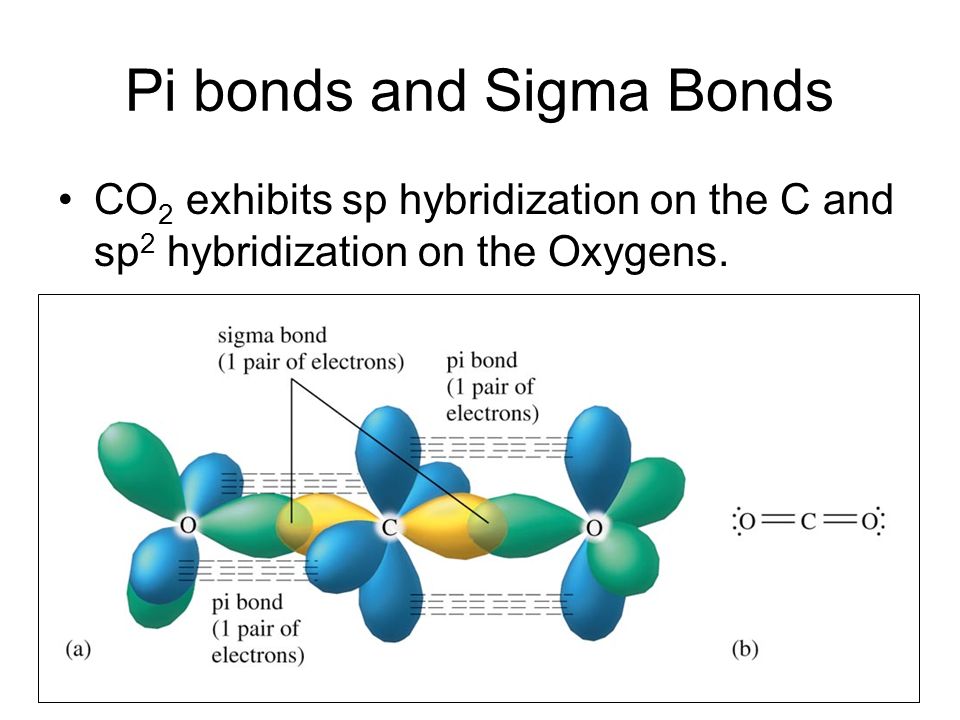
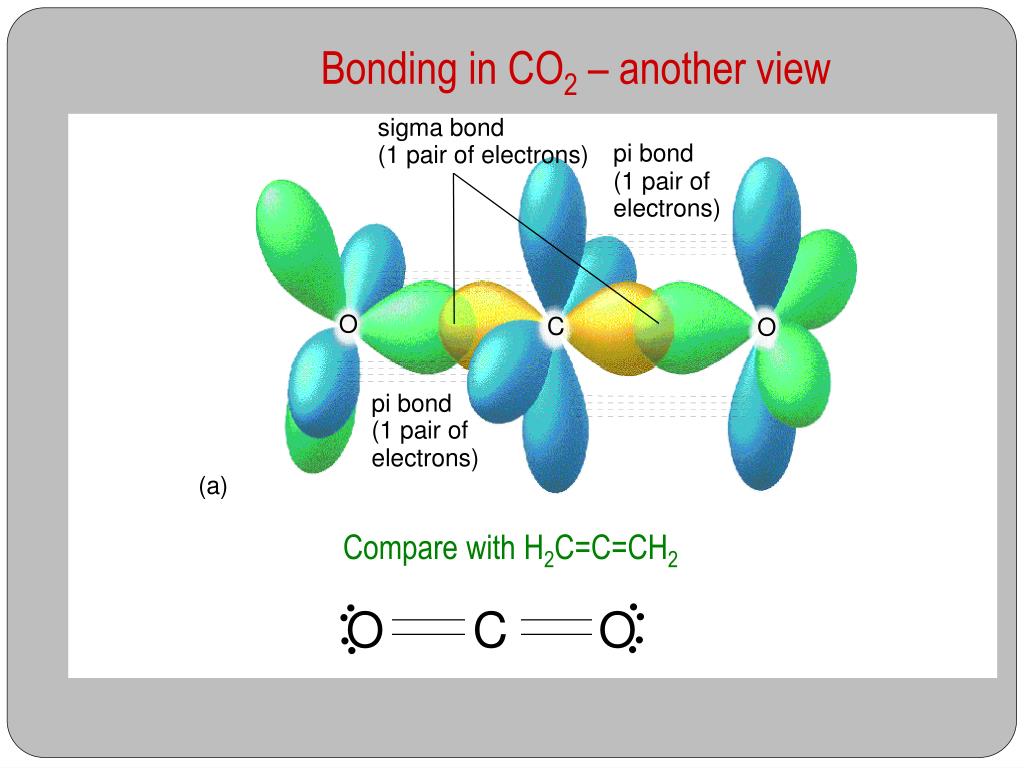
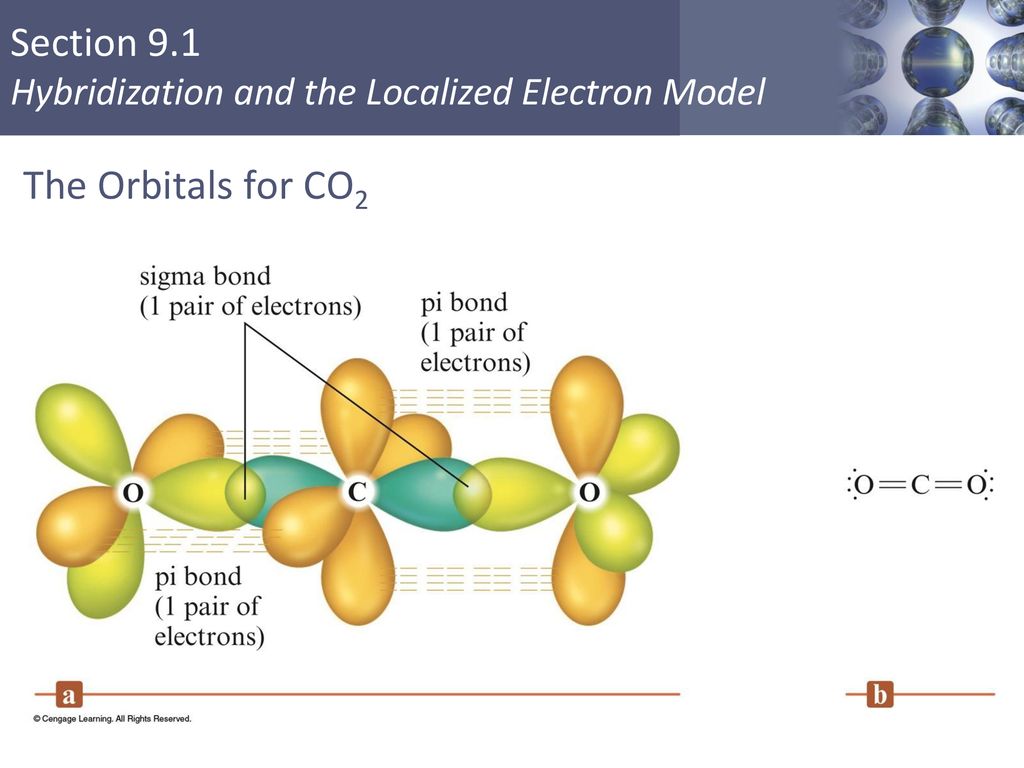
Sigma and Pi Bonds in Co2
Some of the properties of HCN are. Check out a sample QA here.
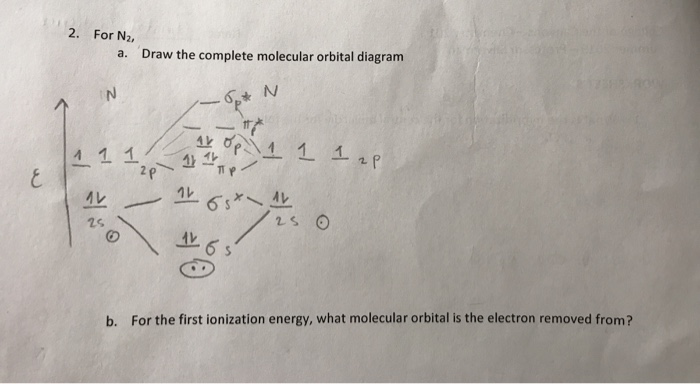
Solved 7 Draw All Orbitals Hybridized And Unhybridized Chegg Com
What atomic or hybrid orbitals make up the sigma.

. TF Pi bonds are stronger than sigma bonds. Carbon dioxide contains two double bonds and we know that each double bond consists of one sigma and one pi bond. A molecule has sp3d2 hybridization.
Nitromethane contains 3 C H sigma bonds 1 C N sigma bond 2 N O sigma bonds and 1 N O pi bond. Usually most people think that C 2 molecule having 8 valence electrons does not exist. Pi bond is formed by natural overlap of p orbitals.
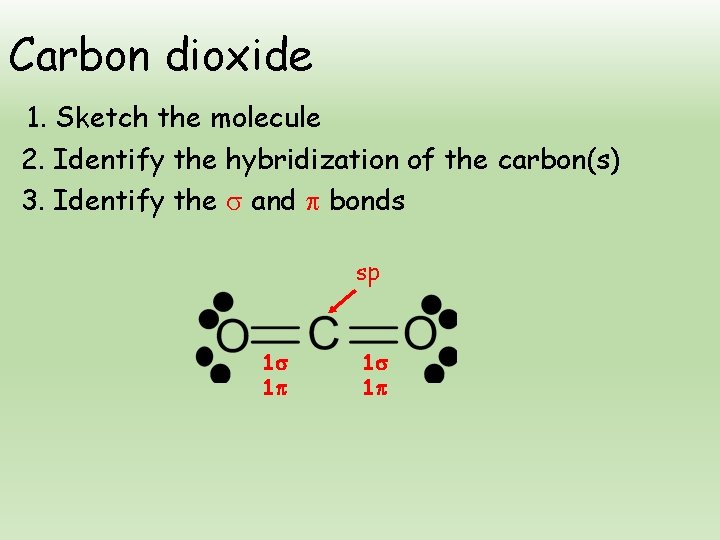
How many sigma and pi bonds does CO2 have. Explain how the sigma and pi bonds are made in CO2. Well i think that there are 5 bonds.
One sigma bond and one Pi bond combine to form a double bond. 2 C H 4 2 N H 3 3 O 2 Δ 1200 C P t 2 H C N 6 H 2 O. Count all the sigma and pi bonds in the following molecules.
Each oxygen makes 1 sigma bond and also needs 2 orbitals for lone pairs of electrons. O2NCH2CH2NO2 CH3COCH3 has CO HNO2 has OH. So the HCN molecule has 2 sigma σ bonds and 2 pi π bonds.
How many bonds are between carbon and oxygen in carbon dioxide. 7 How do you draw a Lewis structure. How many σ sigma and π pi bonds are present in CO 2.
The pi bond is the second bond of the double bonds between the carbon atoms and is shown as an elongated green lobe that extends both above and below the plane of the molecule. Want to see the full answer. Bonding in Carbon Dioxide.
Between the carbon atoms there is one sigma bond and two pi bonds making this a triple bond. In 13-butadiene there are c3 sigma bonds and 2 pi bonds hence the ration is 32 or we can say 151. How many sigma and pi bonds in CO2.
A faster way to determine how many pi bonds the molecule has is to. S-S overlapping One s orbital from each participating atom experiences head-on overlapping along the internuclear axis in this type of overlapping. Therefore the correct option is C.
A molecule has sp hybridization electron pair geometry. 5 How many sigma Σ bonds and pi π bonds are in propene CH2 CHCH3. In carbon dioxide there are 2 sigma and 2 pi bonds hence again the raio is 11.
This plane contains the six atoms and all of the sigma bonds. Three sigma bonds are formed from each carbon atom for a total of six sigma bonds total in the molecule. And there are two oxygen present with carbon thats why the suffix di is used.
Whereas it has a triple bond in C N and hence has two pi π bonds. Count all the sigma and pi bonds in the following molecules. N-methyl formamide contains 2 C N sigma bonds four C H sigma bonds one N H sigma bond one C O sigma bond and one C O pi bond.
2 sigma and 2 pi. From the Lewis structure we can see that the carbon in CO2must make 2 sigma bonds and it has no lone pairs. 8 How many sigma bonds are.
O2NCH2CH2NO2 CH3COCH3 has CO HNO2 has OH Question. In C 2 H 2 HCCHsigma bonds3Pi bonds2In C 2 H 4 H 2 CCH 2 sigma bonds5Pi bonds1Hence the answer for a is 32 and b51. 4 How many sigma and pi bonds are in o2.
2 How many sigma and pi bonds are in a Lewis structure. There are two double bonds in carbon dioxide. 6 How many covalent bonds are there in the molecule with the formula ch2chch3.
But it does exist at very high temperatures and in the gaseous state. This atom will be 2sp hybridized with remaining 2pxand 2pyatomic orbitals. In benzene the total sigma bonds are 12 and pi bonds are 3 hence the ratio is 41.
- It can be synthesized by combining ammonia and methane. How many σ sigma and π pi bonds are present in CO2. Hence 2 π bonds are there in C O 2.
1 sigma and 2 pi. Thus the number of σ and π bonds in C 2 molecule will be zero and two respectively. Check out a sample QA here.
Explain how the sigma and pi bonds are made in CO2. The pi bond is the second bond of the double bonds between the carbon atoms and is shown as an elongated green lobe that extends both above and below the plane of the molecule. The chemical symbol is.
Want to see the full answer. 3 How many sigma bonds are in xef4. Therefore CO2 has 2 pi bonds and 2 sigma bonds.

How Many Pi Bonds Are There In Co2
Hybridization Of Orbitals Sections 9 1 And Ppt Video Online Download

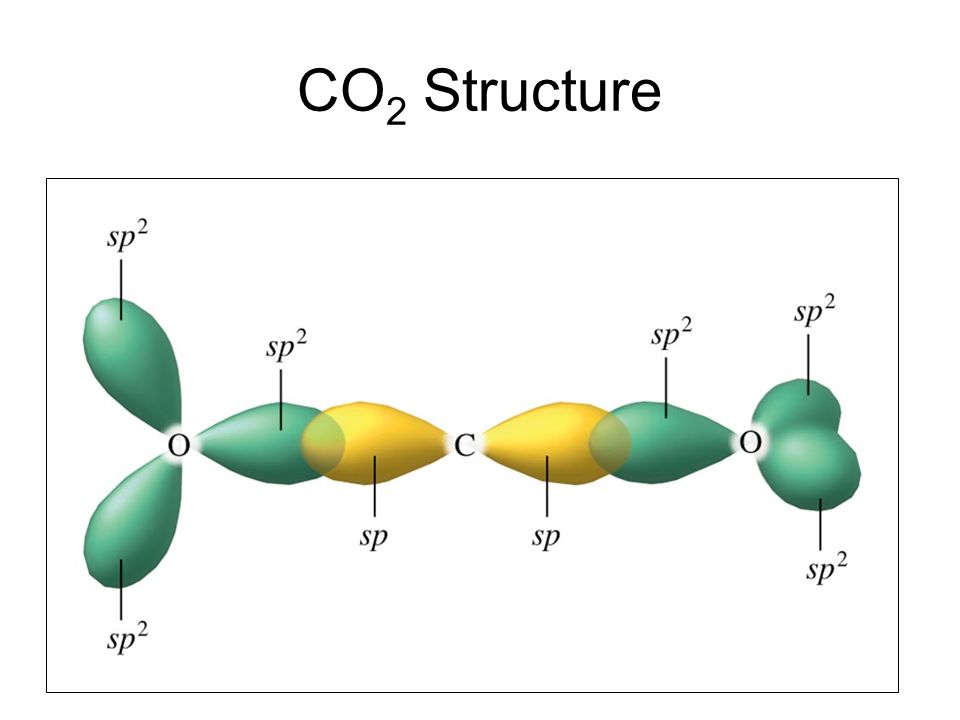
Hybridization Of Co2 Hybridization Of C O In Carbon Dioxide

Orbitals And Covalent Bond Ppt Download

Glv Tutoring In Order To Determine What Type Of Hybridization Is Neccessary For Each Molecule Follow These 3 Easy Steps 1 Determine How Many P Orbitals Will Be Needed P Orbitals Are
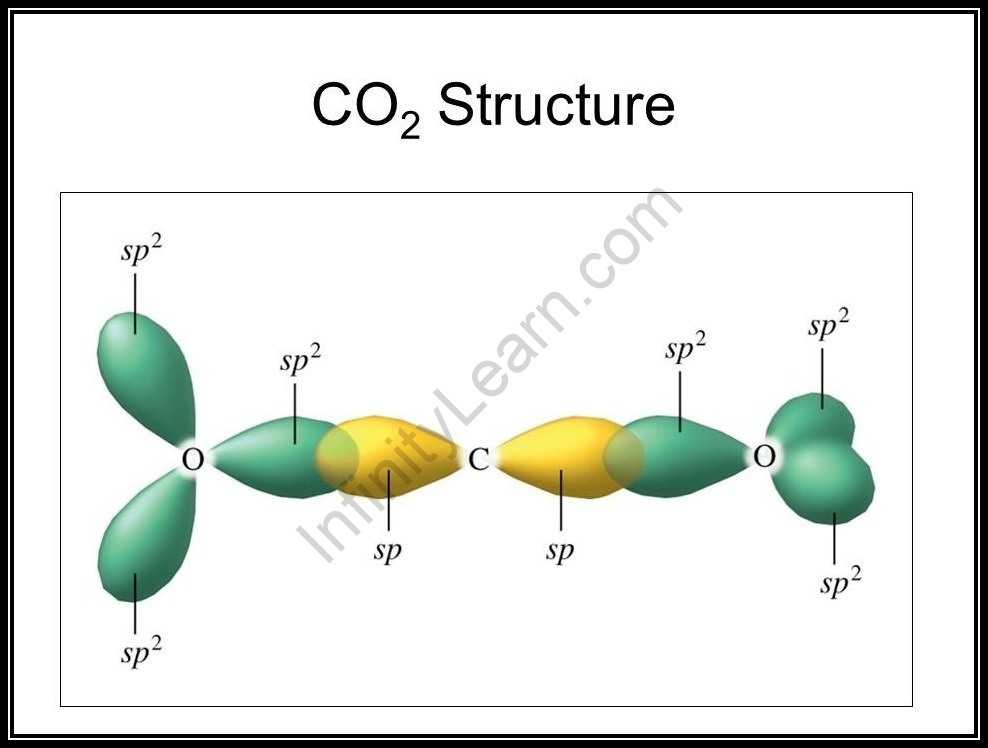
Hybridization Of Carbon Dioxide Infinity Learn
What Is The Hybridization Of Carbon Dioxide Quora
Sigma And Pi Bonds Bonding In Ethylene Bonds
Ppt Chapter 10 Powerpoint Presentation Free Download Id 4569819

S Bonds And Corresponding Hybridization State Download Table
Is There A Picture Of How The Atomic Orbitals Overlapped In Co2 Quora

Bonds And Molecules Prezentaciya Onlajn
Hybridization Of Orbitals Sections 9 1 And Ppt Video Online Download

How Many Pi Bonds Are There In Co2

Hybridization Of Co2 Carbon Dioxide Youtube
Draw The Lewis Structure For Methane Ch4 Ppt Download
Is There A Picture Of How The Atomic Orbitals Overlapped In Co2 Quora
Comments
Post a Comment